The periodic table is one of the most fundamental tools in chemistry, offering a systematic way to understand elements and their properties. Now that we have a solid understanding of electrons, let’s explore how the periodic table is organized and how it helps us predict the characteristics of different elements. In this article, we will discuss its history, structure, and the key terms that will be important throughout your chemistry studies
The History of the Periodic Table
The development of the periodic table was a major milestone in the field of chemistry. Several scientists contributed to its creation, but Dmitri Mendeleev is often credited as its primary architect.
Mendeleev’s Contributions
In the 19th century, both Mendeleev and Lothar Meyer independently published tables that arranged elements by atomic mass. However, Mendeleev is particularly recognized because he used his table to predict the existence of undiscovered elements. He noticed gaps in the table and proposed that elements would eventually be found to fill those spaces. This allowed scientists to estimate the properties of elements before they were officially discovered.
Two notable examples that supported Mendeleev’s predictions were gallium and germanium, which were later discovered and matched his forecasts almost perfectly. This validation solidified the periodic table as an essential tool in chemistry.
The Modern Periodic Table
By the 20th century, scientists realized that elements were better organized by atomic number rather than atomic mass. This discovery refined the periodic table into the structure we use today.
Key Features of the Modern Periodic Table
- Elements are arranged increasingly by atomic number.
- Properties of elements repeat periodically, known as the periodic law.
- The table consists of vertical columns (groups/families) and horizontal rows (periods).
One of the most important shifts in understanding was recognizing that atomic number, not atomic mass, determines the periodic relationships between elements. While atomic masses generally increase across the table, there are exceptions where the atomic number provides a better organizational system.
Timeline of The Periodic Table of Elements With Milestones in its Evolution
| Year | Milestone |
|---|---|
| Prehistoric Times ~6000 BCE | Discoveries of gold and silver |
| Ancient Times ~1500 BCE | Discovery of mercury |
| Late 1700s | Antoine Lavoisier defines an element |
| 1829 | Döbereiner’s triads |
| 1864 | Newlands’ Law of Octaves |
| 1869 | Mendeleev publishes the first periodic table |
| 1870 | Meyer publishes a similar periodic table |
| 1875 | Discovery of gallium supports Mendeleev’s predictions |
| 1894 | Discovery of noble gases (Ramsay and Rayleigh) |
| 1913 | Moseley arranges elements by atomic number instead of atomic mass |
| 1940s | Discovery of transuranium elements begins |
| 1940s | Seaborg’s Actinide series |
| Late 1900s – Early 2000s | Discoveries of synthetic elements |
| 2016 | IUPAC confirmation of elements completing the 7th period |
| 2025 | Modern Periodic Table Continues to Evolve |
Understanding the Structure of the Periodic Table
The periodic table is divided into different sections based on the properties of elements. Understanding these divisions will help you navigate the table efficiently.
1. Groups (Families)
Vertical columns in the periodic table are known as groups or families. Just like members of a family share similar traits, elements in the same group exhibit similar properties.
The four key families you need to remember are:
- Alkali Metals (Group 1)
- Alkaline Earth Metals (Group 2)
- Halogens (Group 17)
- Noble Gases (Group 18)
These families contain some of the most reactive and important elements in chemistry.
2. Periods
Horizontal rows in the periodic table are known as periods. While elements in the same period do not have identical properties, their atomic numbers increase progressively, and their electron configurations follow a predictable pattern.
3. Transition Metals
The middle section of the periodic table consists of transition metals, which behave differently compared to other elements. These elements often have multiple oxidation states and unique properties that set them apart from the main group elements.
Main Group Elements vs. Transition Metals
The periodic table can be broadly divided into two sections:
- Main Group Elements (S and P blocks)
- Found in groups 1, 2, and 13-18.
- Follow predictable chemical trends.
- Commonly involved in chemical reactions.
- Transition Metals (D and F blocks)
- Found in the middle section of the periodic table.
- Exhibit varied and complex behavior.
- Often have multiple oxidation states and form colorful compounds.
Main group elements are relatively predictable, whereas transition metals tend to have exceptions to the rules.
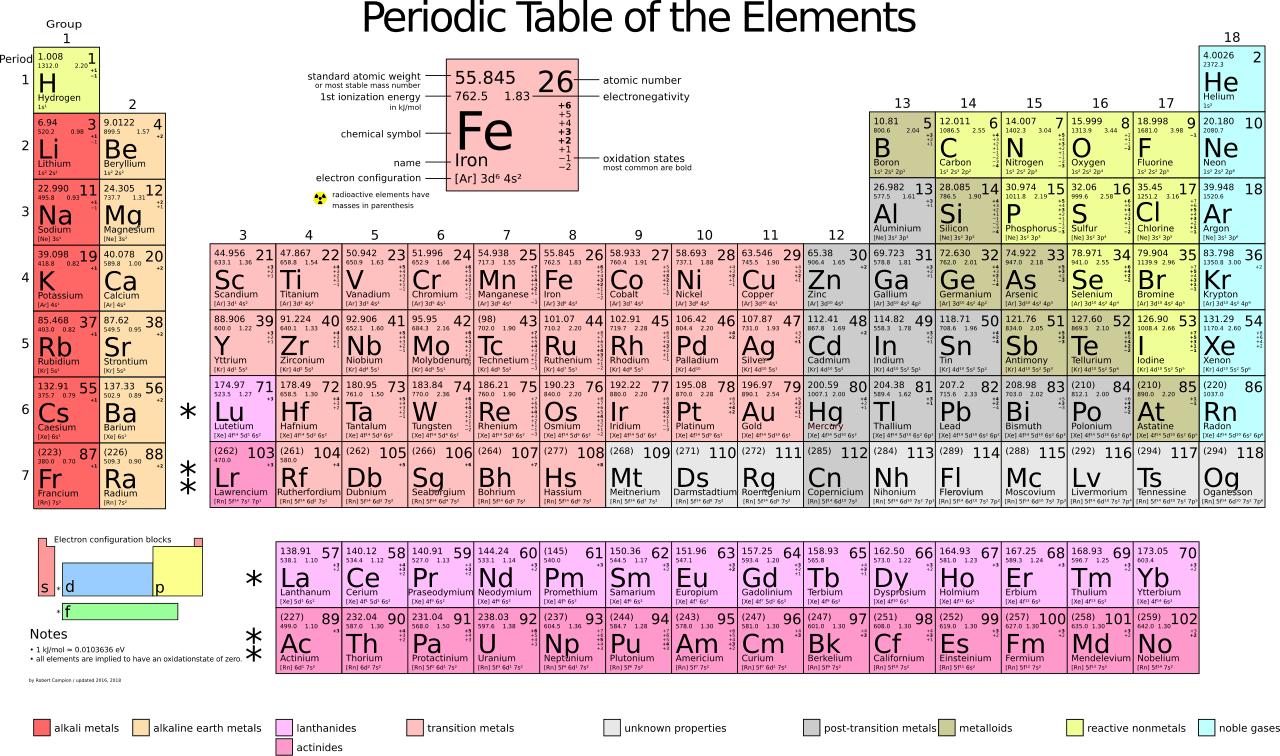
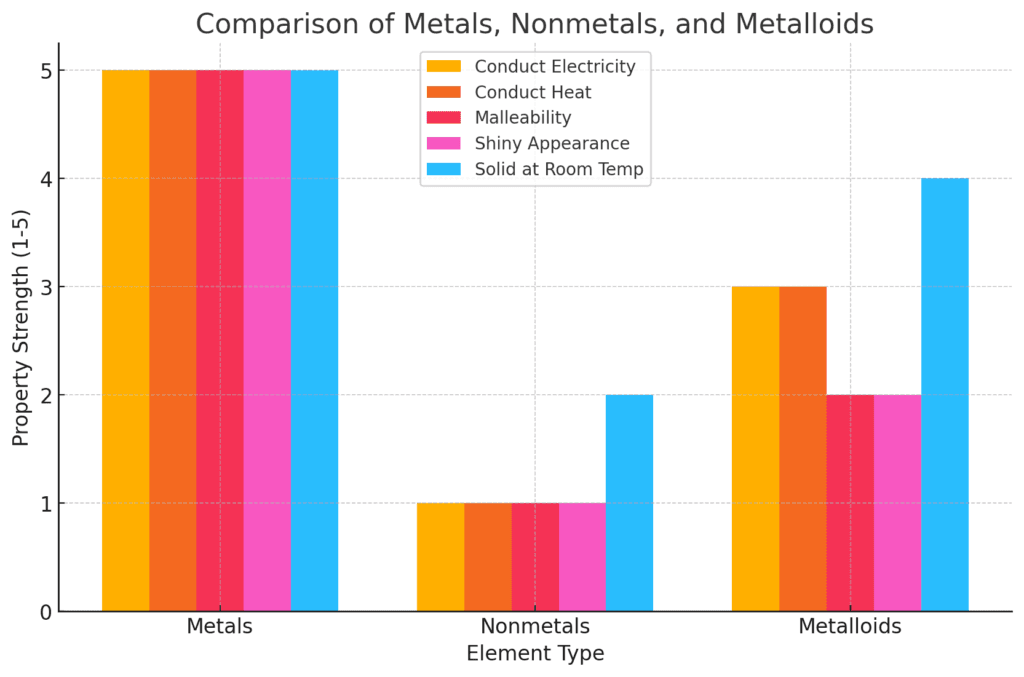
Metals vs. Nonmetals: A Crucial Distinction
One of the most important classifications in chemistry is differentiating between metals and nonmetals. Recognizing these groups quickly will be essential for success in chemical reactions and bonding.
Metals
- Found on the left side of the periodic table.
- Typically shiny, malleable, and conductive.
- Mostly solids at room temperature.
Nonmetals
- Found on the right side of the periodic table.
- Do not conduct electricity or heat well.
- Many exist as gases at room temperature.
Metalloids (Semiconductors)
- Elements that exhibit properties of both metals and nonmetals.
- Located along the stair-step line dividing metals and nonmetals.
- Examples include silicon and boron, which are important in electronics.
Understanding these divisions will make it easier to predict element behavior in reactions and different chemical processes.
Unlocking the Power of the Periodic Table
The periodic table is more than just a chart—it is a powerful tool that helps chemists understand the properties and relationships of elements. From its historical development to its modern organization, the periodic table continues to guide scientific discovery. By familiarizing yourself with its structure, families, and classifications, you’ll be well-prepared for more advanced chemistry topics.
Key Takeaways for Your Chemistry Journey
As you continue your journey learning chemistry, remember these key takeaways from this article:
- Elements are arranged by atomic number.
- Groups share similar properties, while periods show trends.
- Metals, nonmetals, and metalloids have distinct behaviors.
- The periodic table can predict element reactivity and bonding.
- Understanding the table’s layout aids in balancing chemical equations.
- The periodic trends include atomic size, ionization energy, and electron affinity.
Mastering the periodic table will make your chemistry journey smoother and more intuitive. Keep exploring and enjoy discovering the fascinating patterns of the elements!
Share Your Thoughts
Did this article and video help deepen your understanding of the periodic table and its significance in chemistry? Are there any specific topics or questions you’d like us to cover next? Share your opinions, insights, or even your favorite periodic table facts in the comments below. Let’s keep the conversation going—your input helps us create even better content for curious minds like yours!
